Learn How to Transition Adult Patients to a Longer Dosing Interval with INVEGA TRINZA®1
A seamless dosing pathway to INVEGA TRINZA® after adequately treating with INVEGA SUSTENNA® (paliperidone palmitate) for at least 4 months1*
- INVEGA TRINZA® should be administered once every 3 months1
- INVEGA TRINZA® is to be used only after the patient has been adequately treated with 1-month INVEGA SUSTENNA® for at least 4 months1†
- To establish a consistent maintenance dose, it is recommended that the last 2 doses of INVEGA SUSTENNA® be the same dosage strength before starting INVEGA TRINZA®1
- For those who have not taken oral paliperidone, oral risperidone, or injectable risperidone previously, establish tolerability with oral paliperidone or oral risperidone before starting INVEGA SUSTENNA®2
*Based on your evaluation of the patient’s response.
INVEGA TRINZA® Initiation Doses1
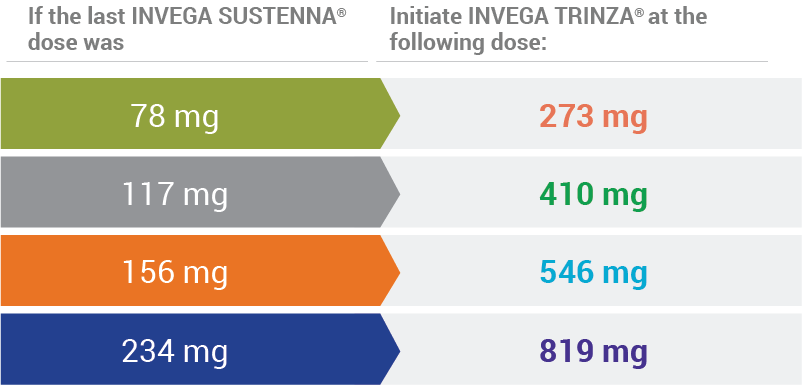
Arrows illustrate the corresponding dose conversion from INVEGA SUSTENNA® to INVEGA TRINZA®.
Conversion from the INVEGA SUSTENNA® 39 mg dose was not studied.
After Transitioning to INVEGA TRINZA®
Following the initial INVEGA TRINZA® dose, INVEGA TRINZA® should be administered once every 3 months.1
If needed, dose adjustments can be made every 3 months in increments within the range of 273 mg to 819 mg based on tolerability or efficacy.1
- Due to the long-acting nature of INVEGA TRINZA®, the patient’s response to an adjusted dose may not be apparent for several months1
Between doses, patients can maintain scheduled treatment plans and routine interactions with their treatment team.
Study Design
A randomized, double-blind, placebo-controlled, long-term maintenance study compared 3-month paliperidone palmitate (INVEGA TRINZA®) with placebo in adult patients with schizophrenia. Patients were treated for 17 weeks with 1-month paliperidone palmitate (INVEGA SUSTENNA®) during an open-label, flexible-dose stabilization phase, and then a single dose of INVEGA TRINZA® during an open-label maintenance phase. This was followed by a fixed dose of INVEGA TRINZA® or placebo once every 3 months in a variable-duration, double-blind phase. A preplanned interim analysis conducted by an Independent Data Monitoring Committee after 42 relapse events resulted in the decision to terminate the study early due to significant efficacy favoring INVEGA TRINZA®. The secondary endpoint of PANSS total score is based on final analysis.1,3
PANSS=Positive and Negative Syndrome Scale.

Download the full Dosing and Administration Guide
References: 1. INVEGA TRINZA® [Prescribing Information]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; August 2021. 2. INVEGA SUSTENNA® [Prescribing Information]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; August 2021. 3. Berwaerts J, Liu Y, Gopal S, et al. Efficacy and safety of the 3-month formulation of paliperidone palmitate vs placebo for relapse prevention of schizophrenia: a randomized clinical trial. JAMA Psychiatry. 2015;72(8):830-839.