Safety and Tolerability Profile of INVEGA TRINZA®
The Overall Safety of INVEGA TRINZA® Was Comparable to INVEGA SUSTENNA® (paliperidone palmitate)1
Most Common Adverse Reactions During the Long-term Maintenance Study1*
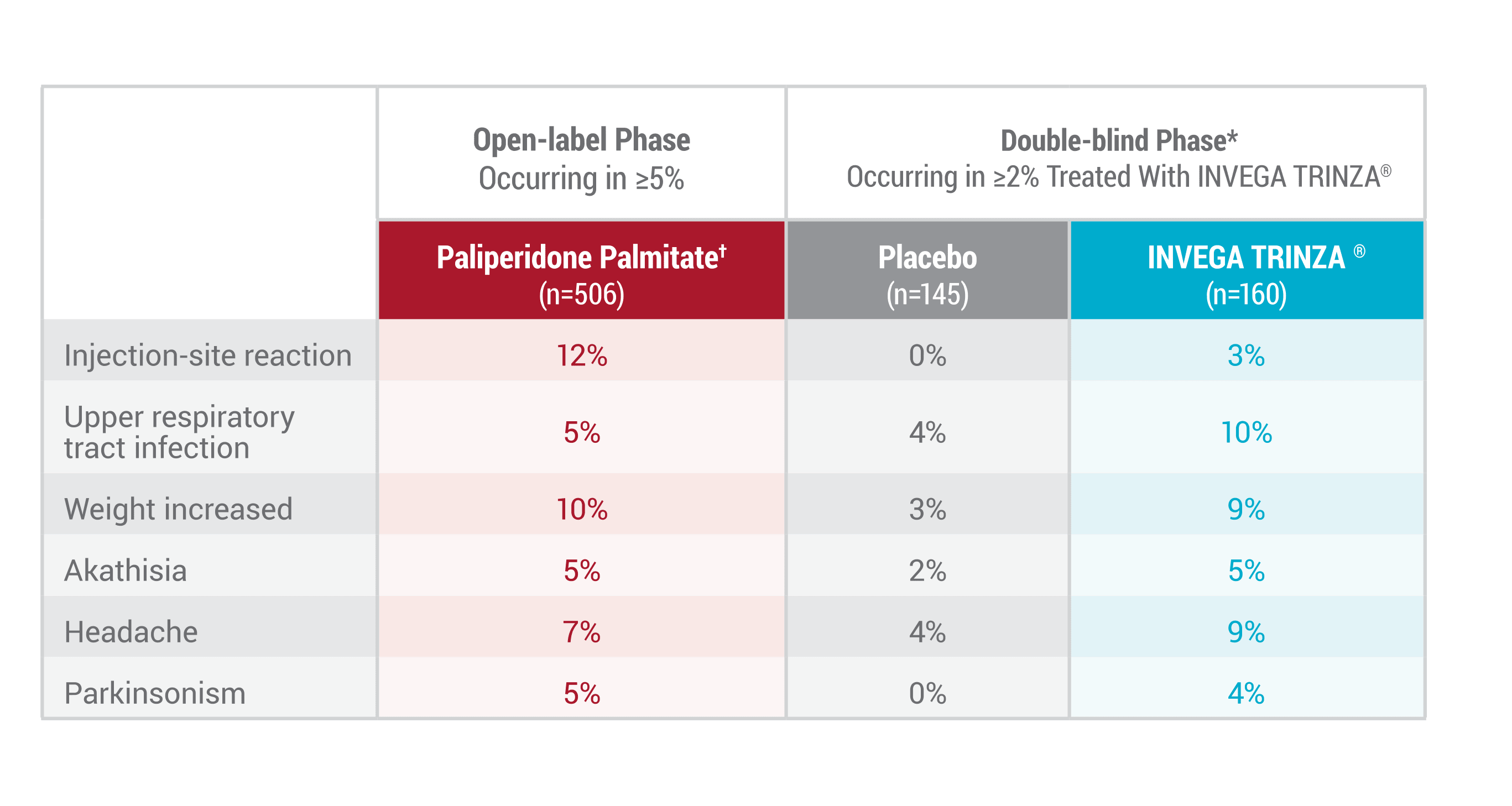
*Occurring in at least 2% in the INVEGA TRINZA® group and at least twice the incidence occurring in the placebo group.1
†During the open-label phase, subjects received several doses of INVEGA SUSTENNA® followed by a single dose of INVEGA TRINZA®.1
- The safety and tolerability of INVEGA TRINZA® was established in a long-term maintenance trial1
- The safety and tolerability of paliperidone has been established in multiple formulations across many clinical trials1-3
WEIGHT GAIN: Significant weight gain has been reported with atypical antipsychotic use. Clinical monitoring of weight is recommended.1
Discontinuation Rates1
- Percentage of patients who discontinued due to adverse reactions during the open-label phase of the trial was 5.1%
- During the double-blind phase, no INVEGA TRINZA®–treated patients and 1 placebo-treated patient discontinued due to an adverse reaction
Other Adverse Reactions
Other Adverse Reactions Identified in the Long-term Maintenance Trial1
- Cardiac disorders: tachycardia
- Gastrointestinal disorders: nausea, vomiting
- Metabolism and nutrition disorders: hyperinsulinemia
- Psychiatric disorders: anxiety
Tardive Dyskinesia
The risk of developing tardive dyskinesia (TD) appears to increase with duration of treatment and total cumulative dose but can develop after relatively brief treatment at low doses. Elderly female patients appeared to be at an increased risk for TD. If signs and symptoms of TD appear in a patient treated with INVEGA TRINZA®, drug discontinuation should be considered.1
Treatment-Emergent Adverse Events (≥5%) During Noninferiority Study4
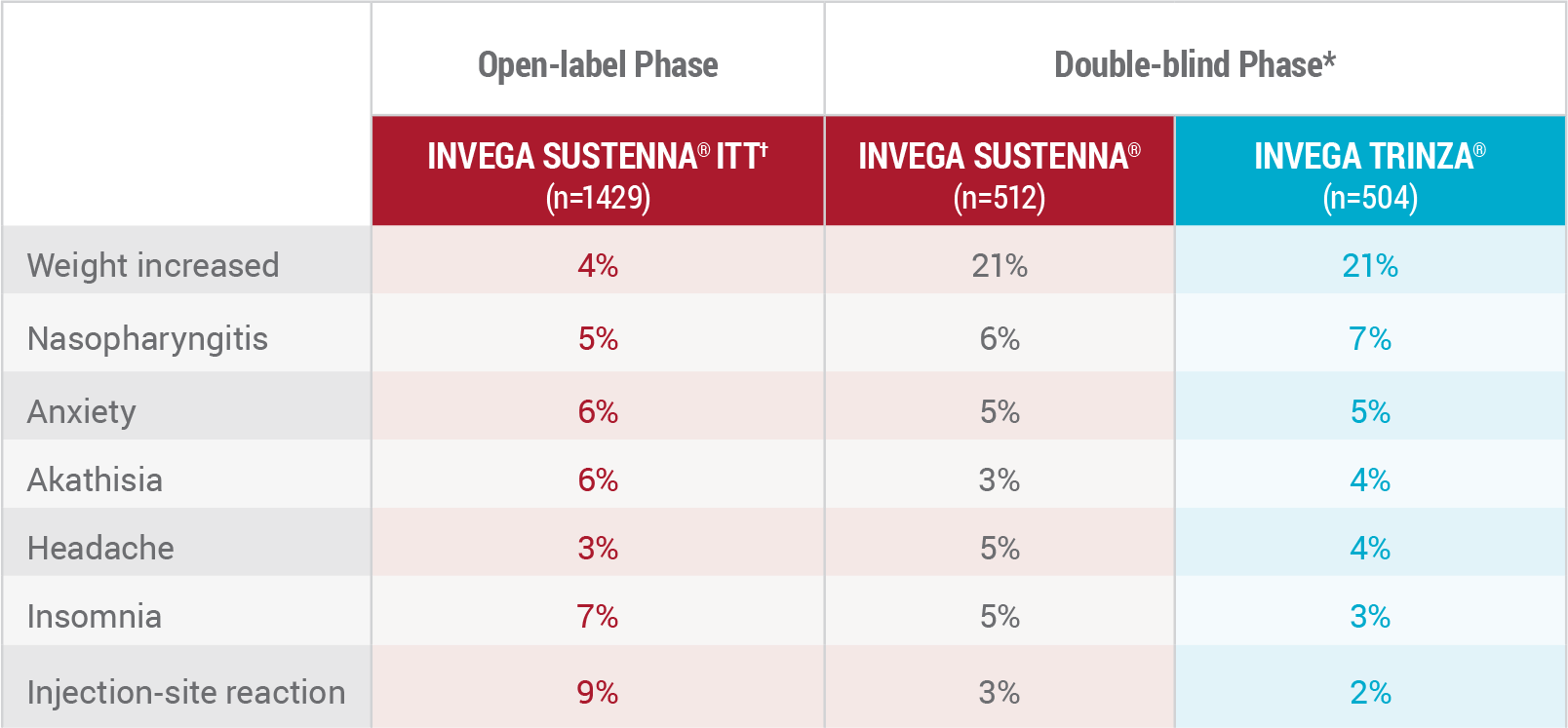
*At least 5% in the open-label phase or in the double-blind INVEGA SUSTENNA® or INVEGA TRINZA® treatment groups.
†ITT=intent-to-treat population, which included all patients enrolled in the open-label phase.
Please see additional Important Safety Information.
Potential INVEGA TRINZA® Drug Interactions
| Concomitant drug name or class | Clinical rationale | Clinical recommendation |
|---|---|---|
| Drugs with potential for inducing orthostatic hypotension | Because INVEGA TRINZA® has the potential for inducing orthostatic hypotension, an additive effect may occur when INVEGA TRINZA® is administered with other therapeutic agents that have this potential. | Monitor orthostatic vital signs in patients who are vulnerable to hypotension. |
| Strong inducers of CYP3A4 and P-gp (eg, carbamazepine, rifampin, or St. John’s wort) | The concomitant use of paliperidone and strong inducers of CYP3A4 and P-gp may decrease the exposure of paliperidone. | Avoid using CYP3A4 and/or P-gp inducers with INVEGA TRINZA® during the 3-month interval, if possible. If administering a strong inducer is necessary, consider managing the patient using paliperidone extended-release tablets. |
| Levodopa and other dopamine agonists | Paliperidone may antagonize the effect of levodopa and other dopamine agonists. | Monitor and manage patient as clinically appropriate. |
CYP3A4=cytochrome P450 3A4; P-gp=P-glycoprotein
References: 1. INVEGA TRINZA® [Prescribing Information]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; August 2021. 2. INVEGA SUSTENNA® [Prescribing Information]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; August 2021. 3. INVEGA® [Prescribing Information]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; March 2022. 4. Savitz AJ, Xu H, Gopal S, et al. Efficacy and safety of paliperidone palmitate 3-month formulation for patients with schizophrenia: a randomized, multicenter, double-blind, noninferiority study. Int J Neuropsychopharm. 2016;19(7):1-14.