Patient claims data
Symphony Health, a robust open-source database, captures prescription information for more than 280 million patients. The database does not capture services received outside of the Symphony Health network.10

FOR PATIENTS ADEQUATELY TREATED WITH INVEGA SUSTENNA® (paliperidone palmitate) FOR AT LEAST 4 MONTHS
With 3 months of symptom control, she’s setting new treatment goals
Actor Portrayal
You've come a long way since your patient has been adequately treated with INVEGA SUSTENNA® for at least 4 months.*
Actor Portrayal
Actor Portrayal

Clinical Guidance Supports Earlier Use of LAIs in Adults With Schizophrenia1
The National Council for Mental Wellbeing† supports the safe and effective use of LAIs by encouraging mental healthcare professionals to utilize LAIs as an earlier treatment option.
†Formerly known as the National Council for Behavioral Health.
LAI=long acting injectable.

A randomized, double-blind, placebo-controlled, long-term maintenance study compared 3-month INVEGA TRINZA® with placebo2,3
A noninferiority study
compared INVEGA TRINZA® to INVEGA SUSTENNA®4

An international, open-label study examined symptomatic remission and goal attainment with INVEGA TRINZA®5,6
One Dose of INVEGA TRINZA® Delivers 3 Months of Sustained Plasma Concentration2,7
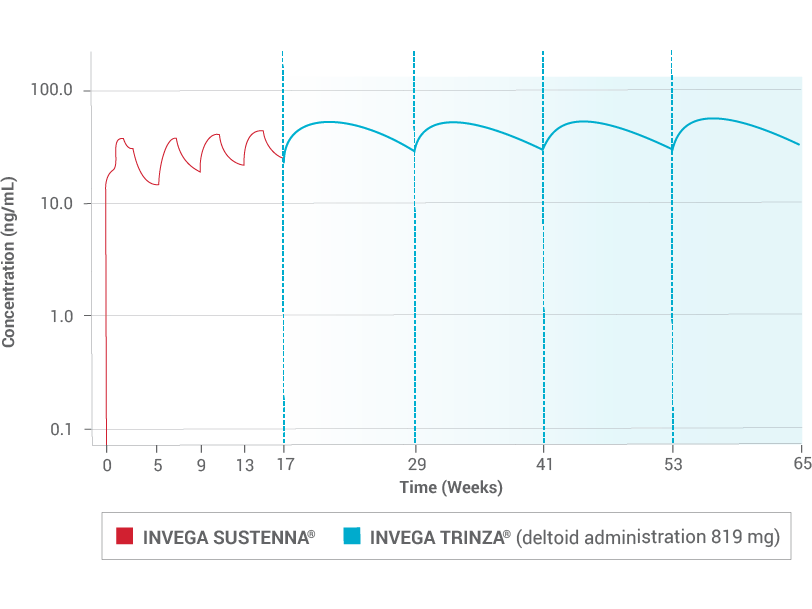
Due to the difference in median pharmacokinetic profiles between the 2 products (INVEGA TRINZA® and INVEGA SUSTENNA®), caution should be exercised when making a direct comparison of their pharmacokinetic properties.

Actor Portrayal
Actor Portrayal

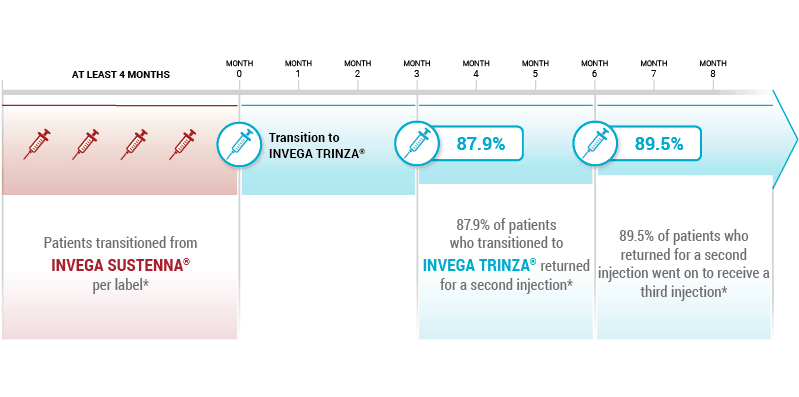
Based on a retrospective cohort analysis of pharmacy and medical claims data, of adult patients with schizophrenia (n=1603), from the Symphony Health Solutions (SHS) database from May 2014 to September 2016. First data on patient claims for INVEGA TRINZA® were from July 2015.
Patient claims data
Symphony Health, a robust open-source database, captures prescription information for more than 280 million patients. The database does not capture services received outside of the Symphony Health network.10
References: 1. National Council for Mental Wellbeing. Guide to Long-Acting Medications for Providers and Organizations. https://www.thenationalcouncil.org/resources/guide-to-long-acting-medications/. Published June 5, 2019. Accessed April 29, 2022. 2. INVEGA TRINZA® [Prescribing Information]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; August 2021. 3. Berwaerts J, Liu Y, Gopal S, et al. Efficacy and safety of the 3-month formulation of paliperidone palmitate vs placebo for relapse prevention of schizophrenia: a randomized clinical trial. JAMA Psychiatry. 2015;72(8):830-839. 4. Savitz AJ, Xu H, Gopal S, et al. Efficacy and safety of paliperidone palmitate 3-month formulation for patients with schizophrenia: a randomized, multicenter, double-blind, noninferiority study. Int J Neuropsychopharm. 2016;19(7):1-14. 5. Garcia-Portilla MP, Llorca PM, Maina G, et al. Symptomatic and functional outcomes after treatment with paliperidone palmitate 3-month formulation for 52 weeks in patients with clinically stable schizophrenia. Ther Adv Psychopharmacol. 2020 May 25;10:2045125320926347. doi: 10.1177/2045125320926347. eCollection 2020. 6. Lambert M, Sanchez P, Bergmans P, et al. Effect of paliperidone palmitate 3-month formulation on goal attainment and disability after 52 weeks’ treatment in patients with clinically stable schizophrenia. Neuropsychiatr Dis Treat. 2020;16:3197-3208. 7. Data on File. Janssen Pharmaceuticals, Inc, Titusville, N.J. 8. Ravenstijn P, Remmerie B, Savitz A, et al. Pharmacokinetics, safety, and tolerability of paliperidone palmitate 3-month formulation in patients with schizophrenia: A phase-1, single-dose, randomized, open-label study. J Clin Pharmacol. 2016;56(3):330-339. 9. Joshi K, Lafeuille MH, Brown B, et al. Baseline characteristics and treatment patterns of patients with schizophrenia initiated on once-every-three-month paliperidone palmitate in the real-world setting. Curr Med Res Opin. 2017;33(10):1763-1772. 10. Symphony Health. What we do. Accessed April 29, 2022. https://symphonyhealth.com/what-we-do