INVEGA TRINZA® Helped Adults With Schizophrenia Achieve Symptomatic Remission1
In a 52-week, International,* Prospective, Single-arm, Open-label Study of 305 Clinically Stable† Adults on INVEGA TRINZA®
*This study was conducted entirely outside of the United States.
†Prior to treatment with INVEGA TRINZA®, all patients had received adequate treatment with INVEGA SUSTENNA® for ≥4 months, with the last 2 doses being the same, indicating clinical stability.
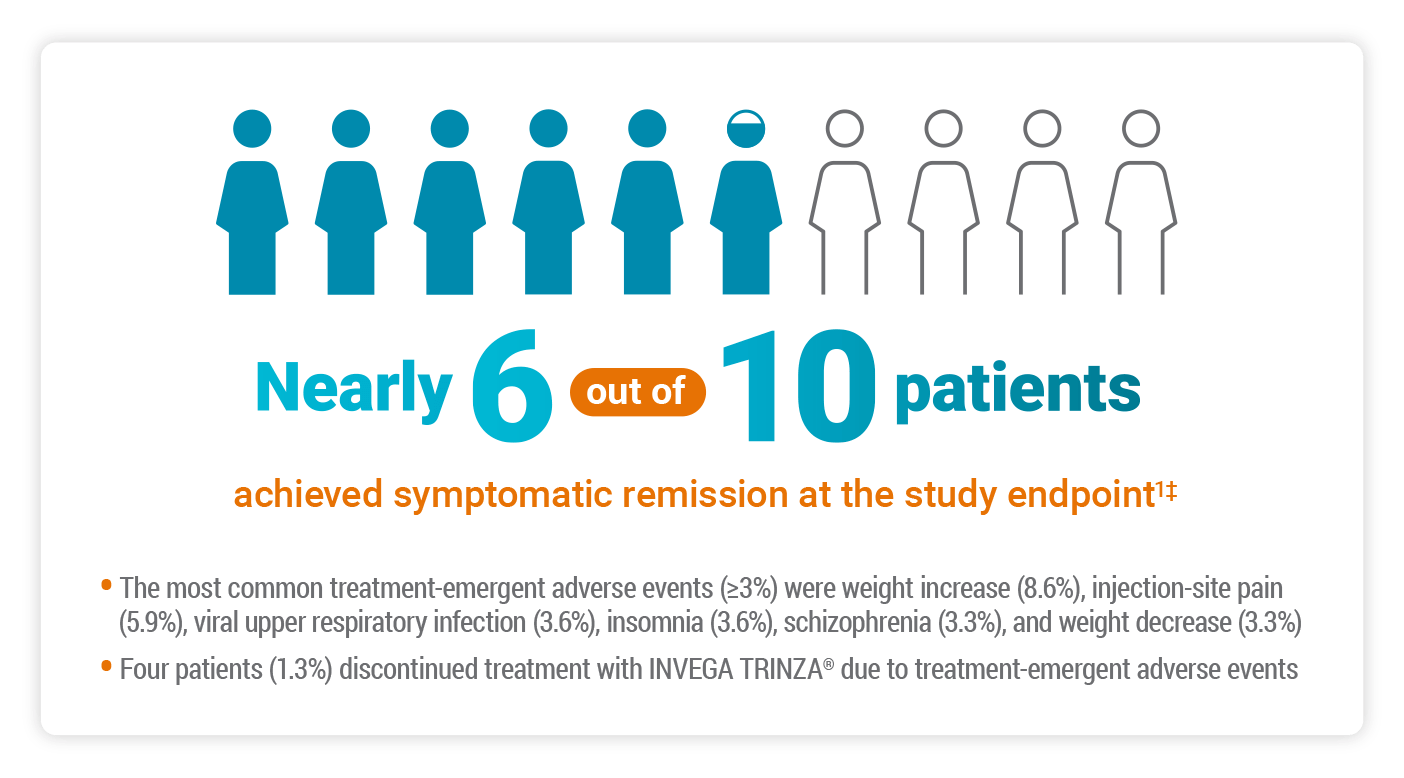
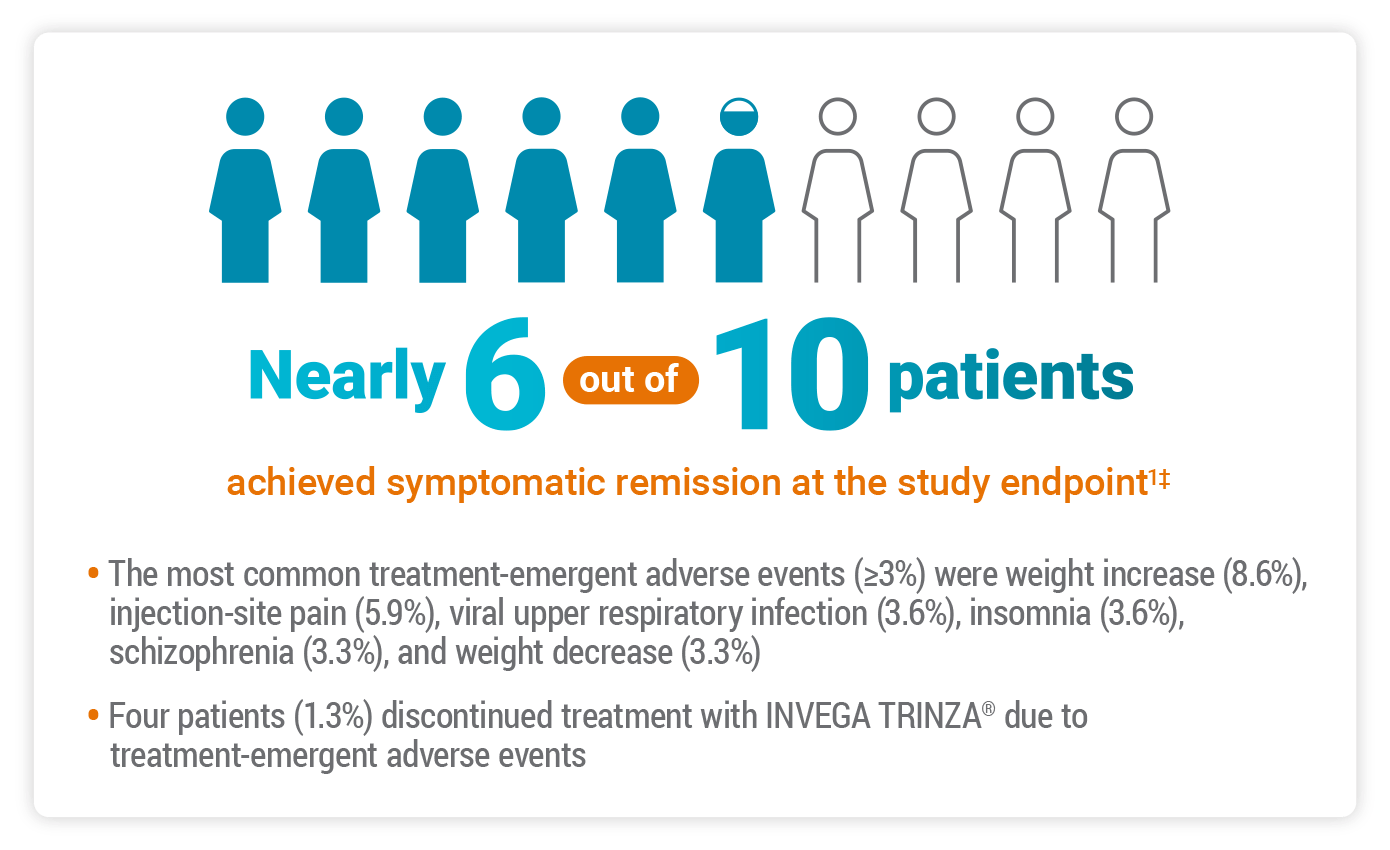
‡56.8% of subjects achieved symptomatic remission as defined by the Andreasen remission criteria, which includes individual scores from all 3 domains of the PANSS.
PANSS=Positive and Negative Syndrome Scale.
Primary Endpoint: Symptomatic Remission in a Real-world Population
The objective of the study was to perform a comprehensive assessment of the efficacy and safety of INVEGA TRINZA® in clinically stable adults with schizophrenia—including patients with concomitant medications and mild-to-moderate substance abuse.1
The primary efficacy endpoint was the proportion of patients who achieved symptomatic remission according to the Andreasen criteria at the last observation carried forward.1
Andreasen et al defined remission as2:
“[…] a state in which patients have experienced an improvement in core signs and symptoms to the extent that any remaining symptoms are of such low intensity that they no longer interfere significantly with behavior and are below the threshold typically utilized in justifying an initial diagnosis of schizophrenia.”
According to the Andreasen remission criteria, symptomatic remission is achieved when the scores for 8 select PANSS items are maintained at ≤3 for at least 6 months.2
Three Months of Symptom Control Can Help Patients Set New Treatment Goals3
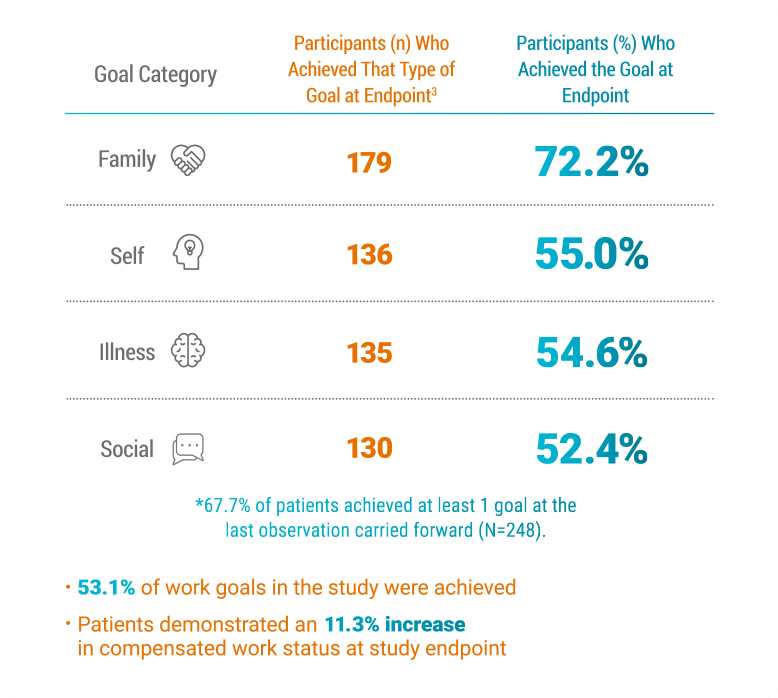
Secondary Endpoint: Goal Attainment
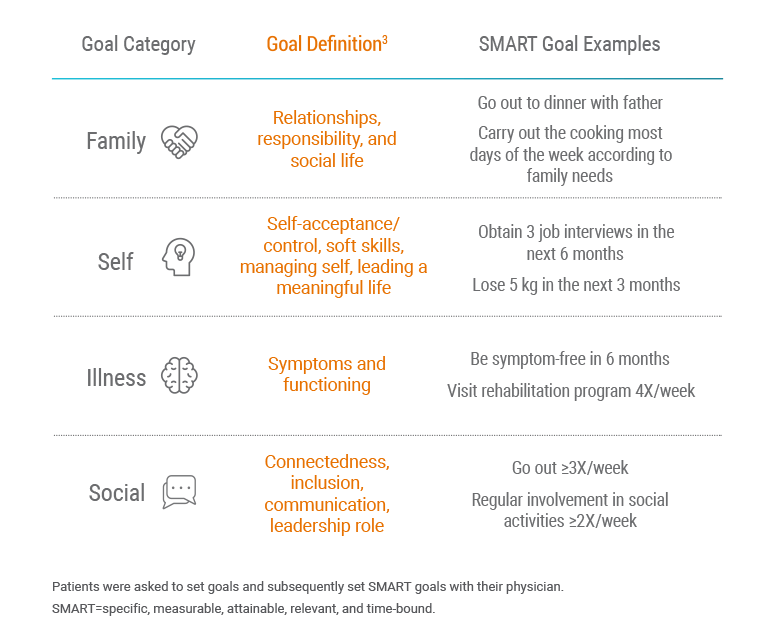
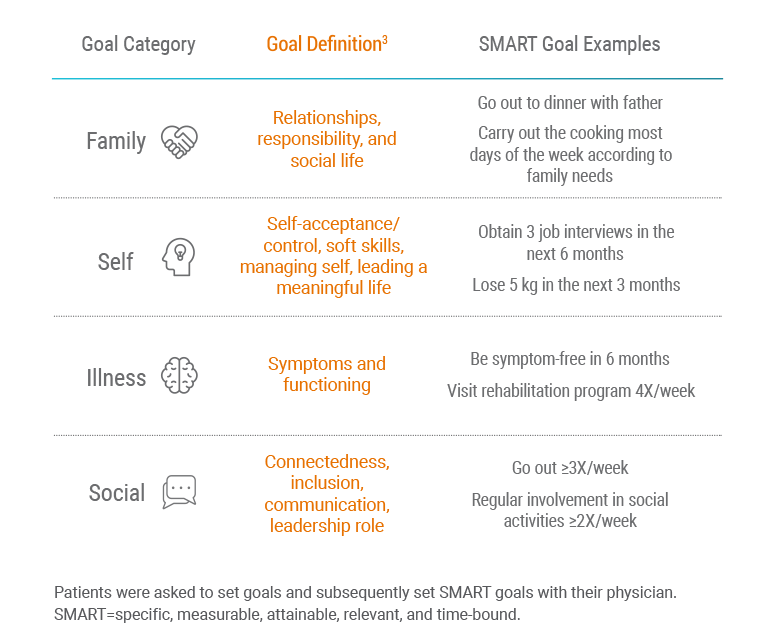
Goals were grouped into 4 categories: family, illness, self, and social.3
- Family goals were the most important to patients
- Self goals were most frequently related to work
Nearly 7 out of 10 Patients Achieved at Least 1 of Their
Personal Goals3*
Family Goals Were the Most Often Achieved3
Individual Patient Goals Were Evaluated Using Goal Attainment Scaling (GAS)
As a secondary endpoint, goal attainment was assessed using GAS to evaluate the impact of transitioning stable patients with schizophrenia from INVEGA SUSTENNA® to INVEGA TRINZA®.3
Goal Attainment Scaling (GAS) Overview3
- GOAL SETTING: 281 of 305 patients provided GAS data at baseline
- GOAL ATTAINMENT: Post-baseline GAS scores were obtained for 248 patients
- Before the first INVEGA TRINZA® administration, each person set up to 3 personal goals
- Each goal was rated for importance, difficulty of achieving, and baseline function
- Goal attainment was evaluated until the goals were either achieved or the study finished (at Month 6, and if relevant, at Month 12 or early withdrawal)
- Each goal was scored on a 5-point scale, recording the degree of achievement for each goal area
- The scale ranged from -2 (much less than expected) to +2 (much better than expected), with goals rated 0 if the patient achieved the expected level
- At baseline, goals were typically scored as -1, unless patients were as bad as they could possibly be in that goal area, in which case they were scored -2
Considerations
Goal Attainment Scale4
Treatment outcomes should not be based solely on the Goal Attainment Scale (GAS) nor should the scale replace other validated clinical measures. Additional reliability and validity data must be gathered on this scale.
Proper selection and scaling of individualized goals are crucial for the validity of the GAS. Results may be subject to bias from the establishment of inaccurate expectation levels (ie, if the expected level of outcomes is underpredicted, the patient's score will be higher. If there is overprediction, the patient's score will be lower).
Study Limitations3
This study was a single-treatment arm, uncontrolled, open-label study, so it did not allow for a direct head-to-head comparison of changes in goal attainment for INVEGA TRINZA® vs INVEGA SUSTENNA®.
The external validity of the study may be limited because patients were stabilized on INVEGA SUSTENNA® before entering the study and thus had a relatively low level of disability. Furthermore, patients with major comorbid psychiatric and severe substance use disorders were excluded from the study. Given that these conditions can impact goal attainment, the study results may not be applicable to this patient group.
Sustained attainment or loss of attainment from Month 6 to Month 12 was not assessed because goals that were achieved at Month 6 were not reassessed at Month 12 according to the protocol.
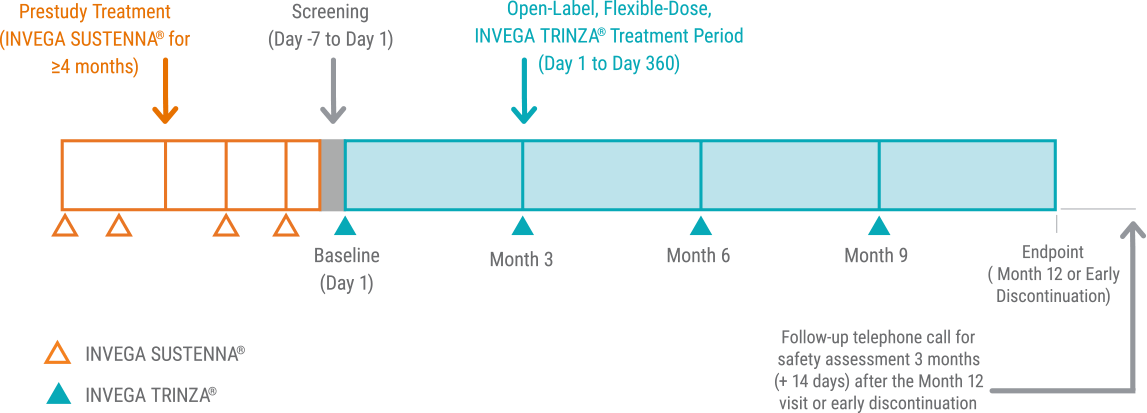
Study Design
Clinically Stable* Adults on INVEGA TRINZA® Were Studied in a Naturalistic† Setting1
291 of 305 patients completed the international, multicenter, prospective, single-arm, open-label, 52-week, Phase IIIb study.1
Patients were generally later in their treatment journey1:
- Average time since diagnosis: 9.2 years
- Average age: 36.5
Length of stabilization on INVEGA SUSTENNA® before treatment with INVEGA TRINZA®1:
- 77.4% >6 months
- 19% 4-6 months
- 3.6% Undefined (≥4 months)‡
*Prior to treatment with INVEGA TRINZA®, all adequately treated patients had received ≥4 months treatment with INVEGA SUSTENNA®, with the last 2 doses being the same, indicating clinical stability.
†Included patients with concomitant medications and mild-to-moderate substance abuse (providing that these patients met study criteria), and allowed flexible dosing after the first administration of INVEGA TRINZA® if required based on clinical judgment.
‡Treatment with INVEGA SUSTENNA® was confirmed to be ≥4 months.
Inclusion Criteria
-
Age (18–50 years)
-
Confirmed DSM-5 diagnosis of schizophrenia
-
Baseline PANSS total score <70
-
Likely to benefit from switching to INVEGA TRINZA® based on their physician’s opinion
-
Received treatment with INVEGA SUSTENNA® (78 to 234 mg) for ≥4 months (with the last 2 doses being the same, in order to ensure that they were stabilized on INVEGA SUSTENNA® and establish an appropriate dose of INVEGA TRINZA®)
Exclusion Criteria
- A DSM-5 diagnosis of other psychiatric disorders or severe substance use disorder within 6 months of screening (patients with mild or moderate substance abuse were not excluded)
- A psychiatric diagnosis secondary to drug abuse, medication, or a general medical condition (eg, clinically notable hypothyroidism, organic brain disorder)
- A serious unstable medical condition (eg, with clinically relevant laboratory abnormalities)
- An imminent risk of suicide
- Experience of intolerable side effects with INVEGA SUSTENNA®
- Stabilization on INVEGA SUSTENNA® 39 mg eq (owing to there being no corresponding dose of INVEGA TRINZA®)
- The use of any long-acting injectable antipsychotic other than INVEGA SUSTENNA® in the 4 months prior to the first administration of INVEGA TRINZA®
- Treatment with clozapine within 3 months of screening
- A history of lack of response or known hypersensitivity to risperidone or paliperidone
- A history or current symptoms of tardive dyskinesia or neuroleptic malignant syndrome
DSM-5=Diagnostic and Statistical Manual of Mental Disorders, 5th ed.
References: 1. Garcia-Portilla MP, Llorca PM, Maina G, et al. Symptomatic and functional outcomes after treatment with paliperidone palmitate 3-month formulation for 52 weeks in patients with clinically stable schizophrenia. Ther Adv Psychopharmacol. 2020 May 25;10:2045125320926347. doi: 10.1177/2045125320926347. eCollection 2020. 2. Andreasen NC, Carpenter WT, Kane JM, et al. Remission in schizophrenia: proposed criteria and rationale for consensus. Am J Psychiatry. 2005;162(3):441-449. 3. Lambert M, Sanchez P, Bergmans P, et al. Effect of paliperidone palmitate 3-month formulation on goal attainment and disability after 52 weeks’ treatment in patients with clinically stable schizophrenia. Neuropsychiatr Dis Treat. 2020;16:3197-3208. 4. Beidel DC, Turner SM, Bellack AS, et al. Using the goal attainment scale to measure treatment outcome in schizophrenia. Int J Partial Hosp. 1983;2(1):33-41.