A Noninferiority Study Compared INVEGA TRINZA® to INVEGA SUSTENNA® (paliperidone palmitate)
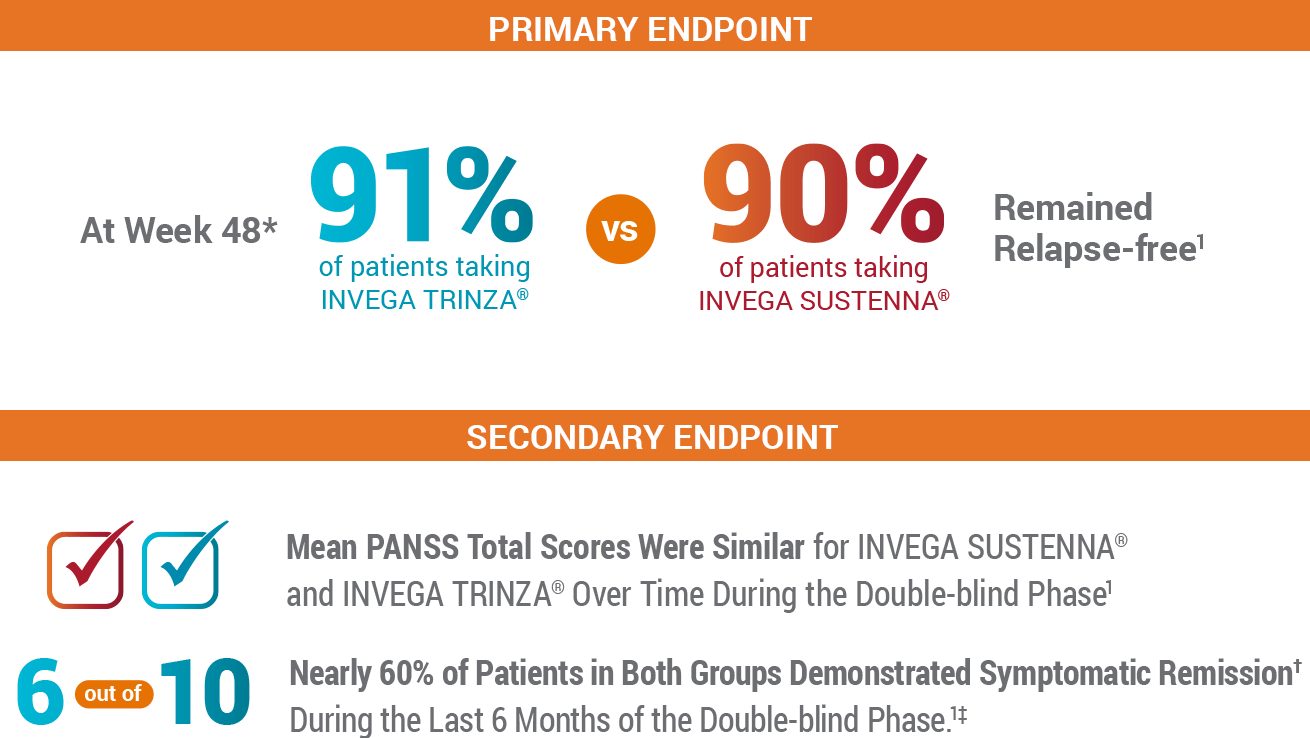
In the Study, INVEGA TRINZA® Delayed Relapse as Effectively as INVEGA SUSTENNA®1
- During the double-blind phase, 3% of patients in each group discontinued treatment due to adverse events
- The most common treatment-emergent AEs (≥5%) in either group during the double-blind phase were increased weight (21% in both groups), nasopharyngitis (INVEGA TRINZA®: 7%; INVEGA SUSTENNA®: 6%), anxiety (5% in both groups), and headache (INVEGA TRINZA®: 4%; INVEGA SUSTENNA®: 5%)
*Results of a 48-week, randomized, double-blind noninferiority trial. The prespecified noninferiority margin was -15%. After screening (<3 weeks) and a 17-week, open-label phase of treatment with INVEGA SUSTENNA®, clinically stable patients were randomized (1:1) to treatment with INVEGA SUSTENNA® or INVEGA TRINZA® for a 48-week double-blind phase. Based on changes from double-blind baseline.
†With one excursion allowed.
‡Symptomatic remission was defined by the Andreasen remission criteria.1
PANSS=Positive and Negative Syndrome Scale.
Abbreviated Study Design
48-Week Randomized, Double-blind Noninferiority Trial1
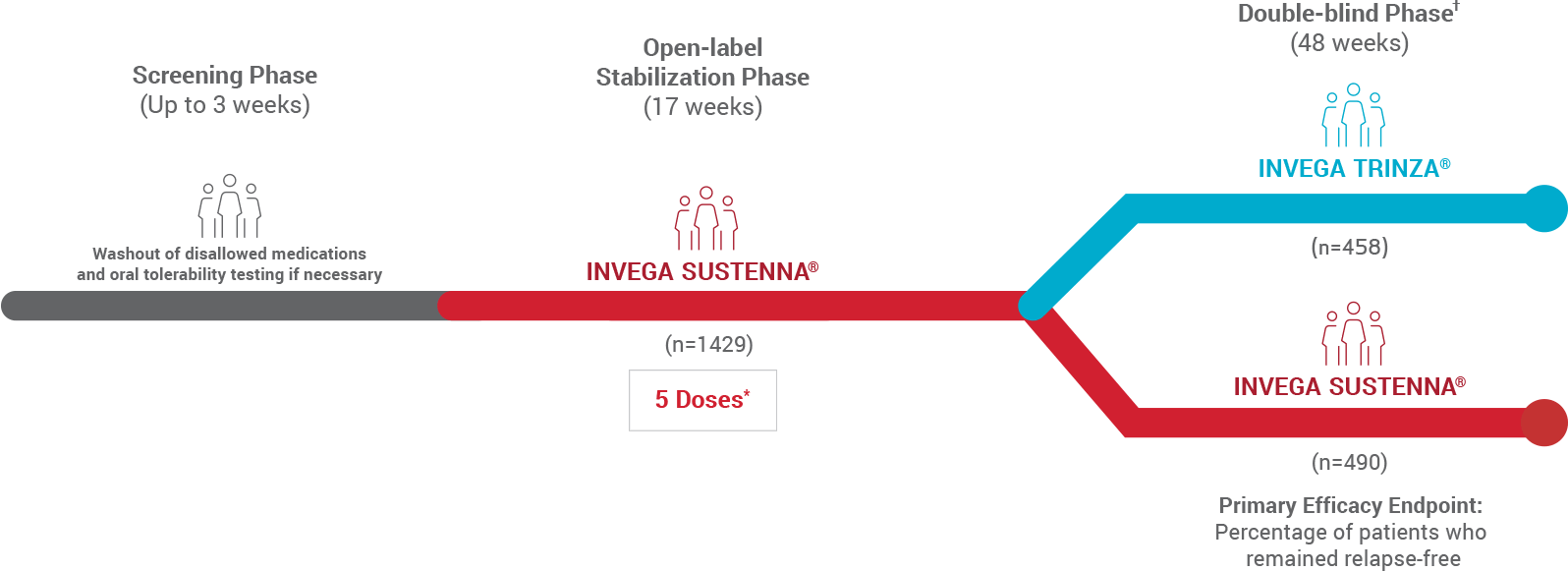
- The prespecified noninferiority margin was -15%
- After a 3-week screening period for oral tolerability testing/washout, patients entered a 17-week open-label stabilization phase (5 doses of INVEGA SUSTENNA®). Clinically stable patients (n=1016) were then randomized in the double-blind phase to continue receiving INVEGA SUSTENNA® or start on INVEGA TRINZA®
*The 5 doses included the 2 initiation doses and 3 maintenance doses.
†Patients successfully stabilized on INVEGA SUSTENNA® were randomized to continue receiving INVEGA SUSTENNA® or to transition to INVEGA TRINZA®.
In the Primary Endpoint, INVEGA TRINZA® Was Noninferior to INVEGA SUSTENNA® After the 48-Week Double-blind Phase1
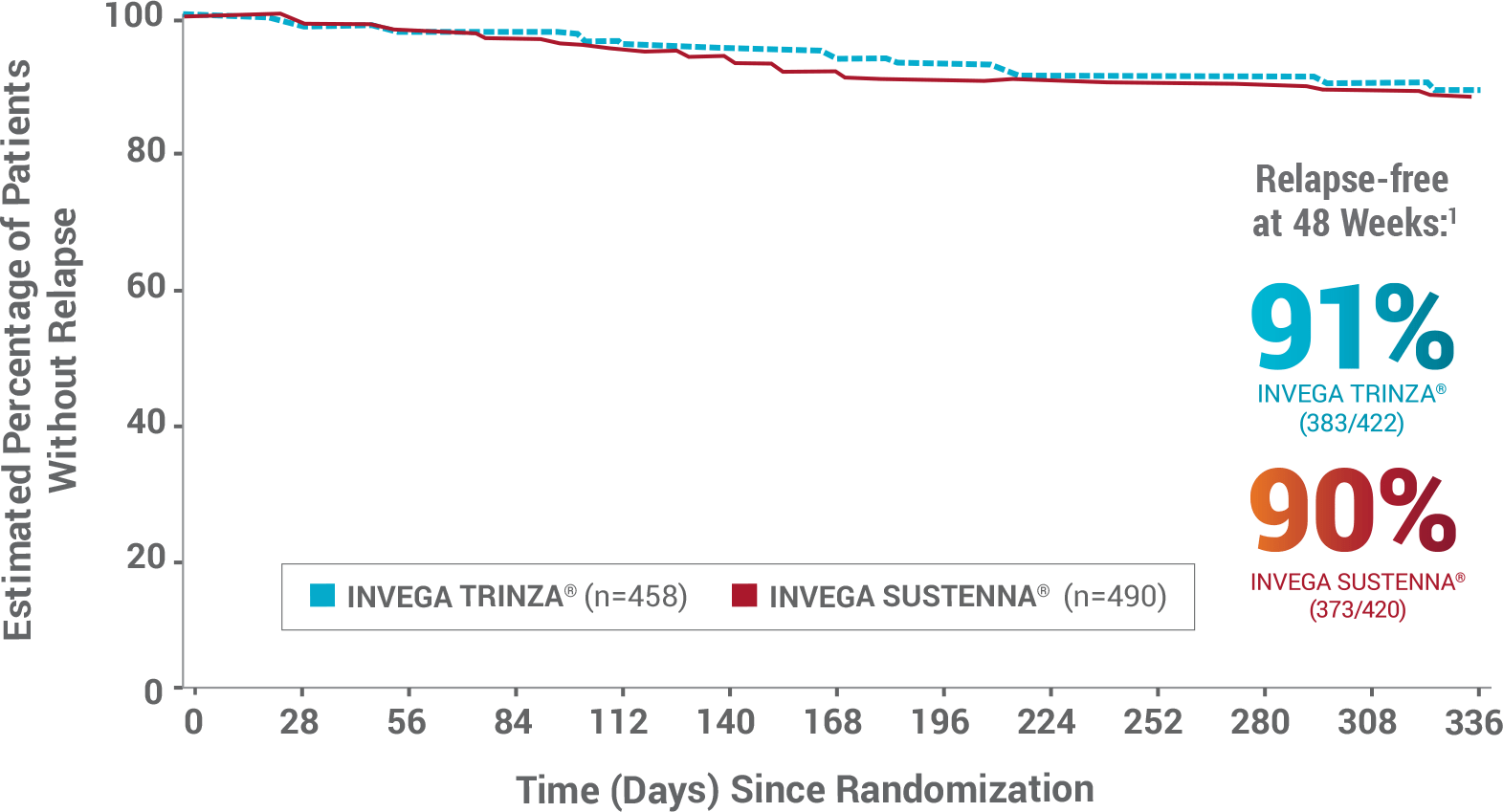
- During the double-blind phase, 3% of patients in each group discontinued treatment due to AEs1
- The most common treatment-emergent AEs (≥5%) in either group during the double-blind phase were increased weight (21% in both groups), nasopharyngitis (INVEGA TRINZA®: 7%; INVEGA SUSTENNA®: 6%), anxiety (5% in both groups), and headache (INVEGA TRINZA®: 4%; INVEGA SUSTENNA®: 5%)
As a Secondary Endpoint, Both Groups Demonstrated Similar Improvements in Clinically Stable* Patients1
INVEGA TRINZA® Achieved Similar Improvements as INVEGA SUSTENNA® (paliperidone palmitate) in Mean PANSS Total Scores in the Double-blind Phase1
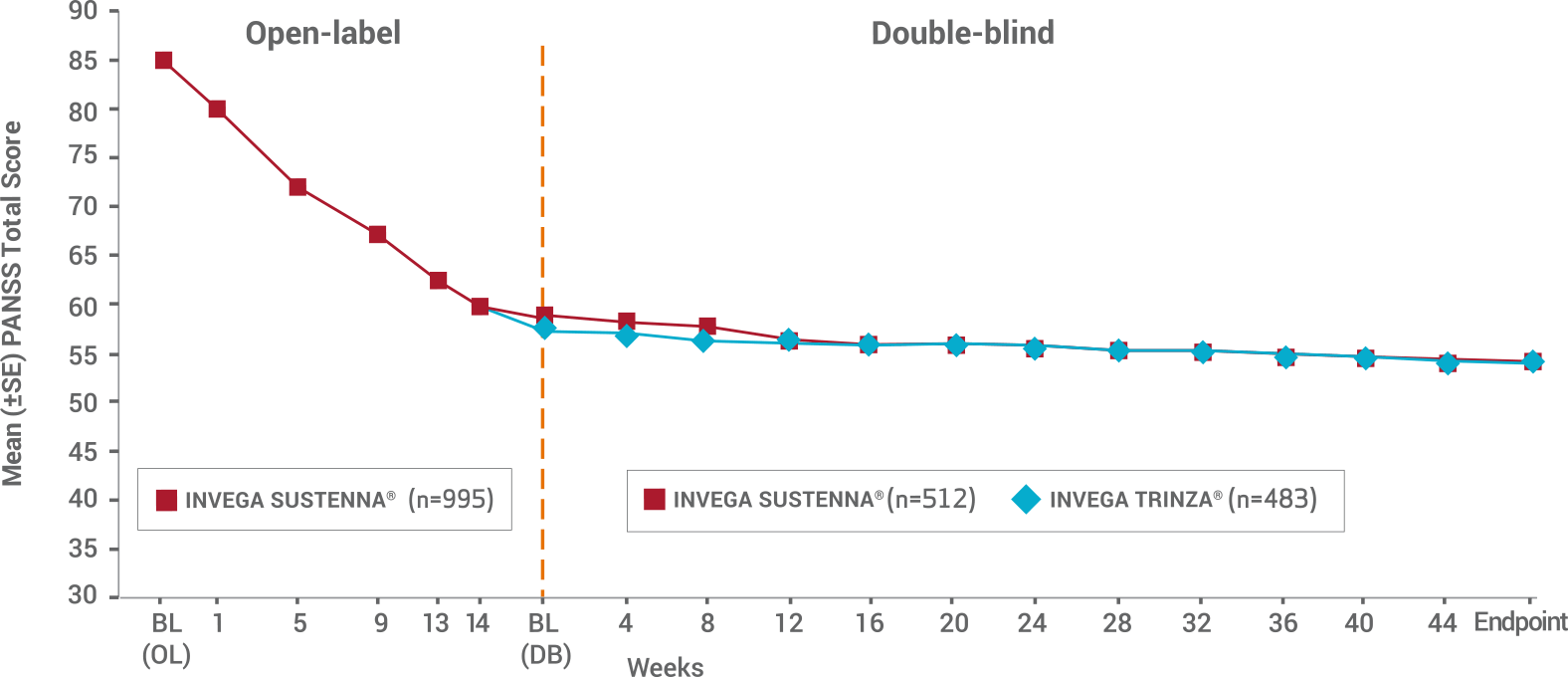
- Double-blind phase: Only patients who were clinically stable* entered the double-blind phase—and they still reported continued improvements in both treatment groups
- The mean baseline PANSS total score for patients entering the double-blind phase in the INVEGA SUSTENNA® group was 58.1, and these patients saw an improvement of -4.3. Patients entering the double-blind phase in the INVEGA TRINZA® group had a mean baseline score of 57.4 and saw an improvement of -3.5
*Defined as PANSS total score <70, PANSS item [P1, P2, P3, P6, P7, G8, G14] scores ≤4, and reduction in Clinical Global Impression-Severity (CGI-S) score by ≥1 from OL baseline.
Patients Showed Symptomatic Remission During the Last 6 Months of the Double-blind Phase1
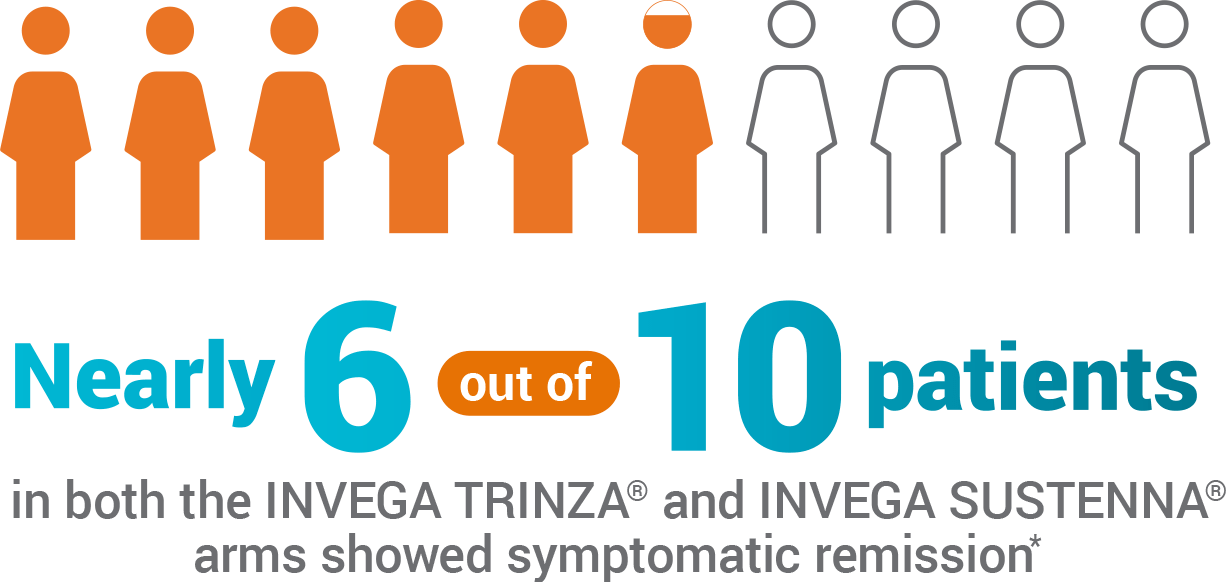
- 59% of patients taking INVEGA SUSTENNA® and 58% of patients taking INVEGA TRINZA® showed symptomatic remission for the last 6 months of the double-blind phase
- Symptomatic remission was defined by the Andreasen remission criteria
*With one excursion allowed.
Study Criteria
Key Inclusion Criteria
- Adult patients (men and women, ages 18-70 years) with a diagnosis of schizophrenia (according to the DSM-IV®), a Positive and Negative Syndrome Scale (PANSS) total score between 70 and 120 at screening and baseline, and worsening of symptoms
- Patients who discontinued other antipsychotics due to insufficient efficacy, safety, or tolerability issues with current therapy, or with preferences for injectable medications
- Women included in the study were postmenopausal, surgically sterile, or used adequate contraception, and eligible men used adequate contraception
Key Exclusion Criteria
- Active DSM-IV® diagnosis other than schizophrenia
- Significant risk of suicidal behavior
- History of substance dependence within 6 months before screening
- Involuntary status in a psychiatric hospital at screening
- History of neuroleptic malignant syndrome, tardive dyskinesia, or any unstable or significant medical or neurological illness
- Morbid obesity (BMI >40 kg/m2)
- Other systemic disease, mental retardation, risk factors for prolonged QT interval, torsades de pointes, or sudden death
- History of intolerability, hypersensitivity, or lack of response to risperidone or paliperidone
- Taking any long-acting injectable antipsychotics within 4 weeks before screening
Definition of Relapse
Occurrence of 1 or more of the following:
- Hospitalization for schizophrenia symptoms (involuntary or voluntary admission)
- 25% increase in Positive and Negative Syndrome Scale (PANSS) total score from randomization on 2 consecutive assessments between 3 and 7 days apart for patients scoring >40 at randomization, or a 10-point increase of patients scoring ≤40 at randomization
- Increase in distinct PANSS item scores (P1, P2, P3, P6, P7, or G8) for 3 consecutive assessments between 3 and 7 days apart
- Clinically significant, deliberate self-injury or violent behavior resulting in suicide, injury, or significant damage
- Suicidal or homicidal ideation and aggressive behavior
Reference: 1. Savitz AJ, Xu H, Gopal S, et al. Efficacy and safety of paliperidone palmitate 3-month formulation for patients with schizophrenia: a randomized, multicenter, double-blind, noninferiority study. Int J Neuropsychopharm. 2016;19(7):1-14.