INVEGA TRINZA® Metabolic Data Observed
Metabolic Change Data: Long-term Maintenance Trial1
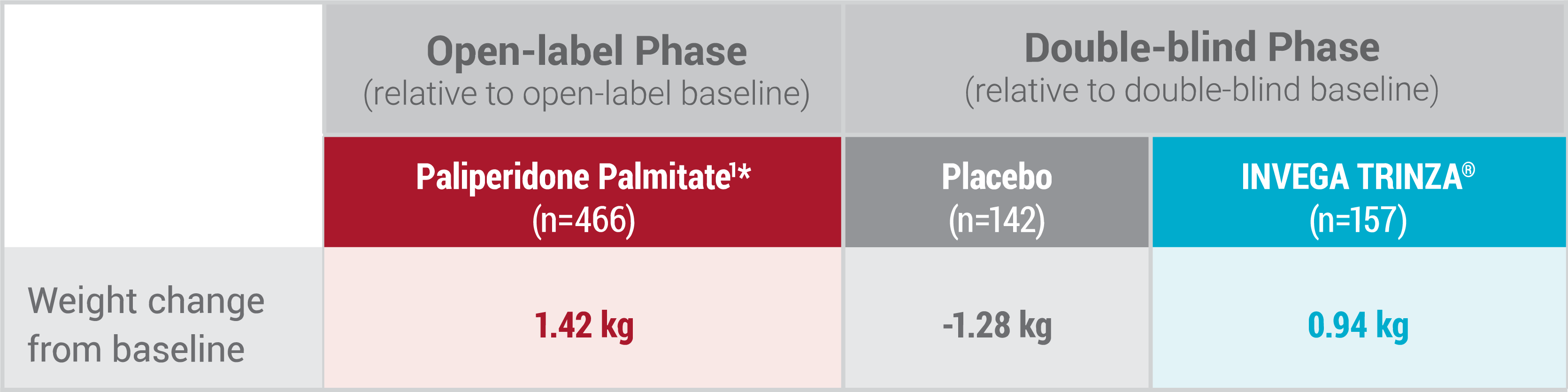
*During the open-label phase, patients received several doses of INVEGA SUSTENNA® (paliperidone palmitate) followed by a single dose of INVEGA TRINZA®.
WEIGHT GAIN: Weight gain has been reported with atypical antipsychotic use. Clinical monitoring of weight is recommended.1
Fasting Glucose1
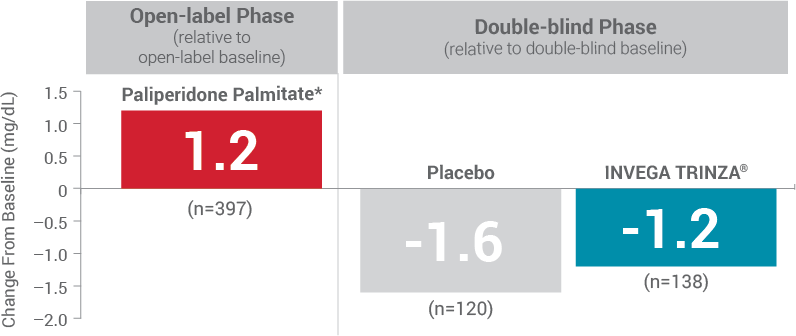
*During the open-label phase, patients received several doses of INVEGA SUSTENNA® followed by a single dose of INVEGA TRINZA®.
Hyperglycemia and Diabetes Mellitus1
- Monitor for symptoms of hyperglycemia including polydipsia, polyuria, polyphagia, and weakness
- Monitor glucose regularly in patients with diabetes or at risk for diabetes
Fasting Lipids1
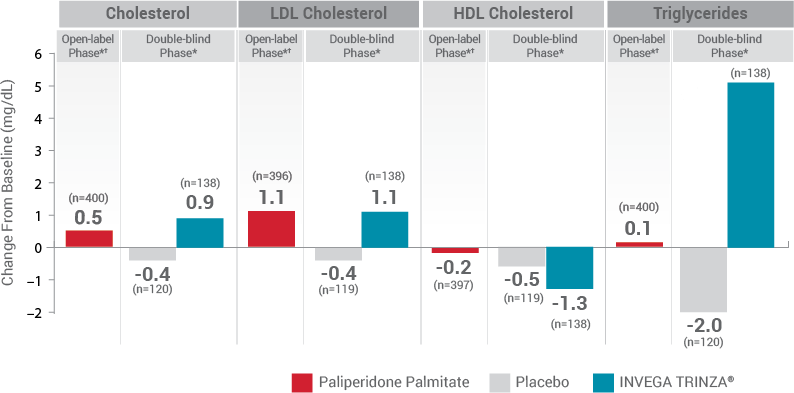
DYSLIPIDEMIA: Undesirable alterations in lipids have been observed in patients treated with antipsychotics.1
Please see additional Important Safety Information below.
*Values for the open-label phase and the double-blind phase are relative to their respective baselines.
†During the open-label phase, patients received several doses of INVEGA SUSTENNA® followed by a single dose of INVEGA TRINZA®.
Reference: 1. INVEGA TRINZA® [Prescribing Information]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; August 2021.