INVEGA TRINZA® Prolactin Data
Observed in the Double-blind Phase
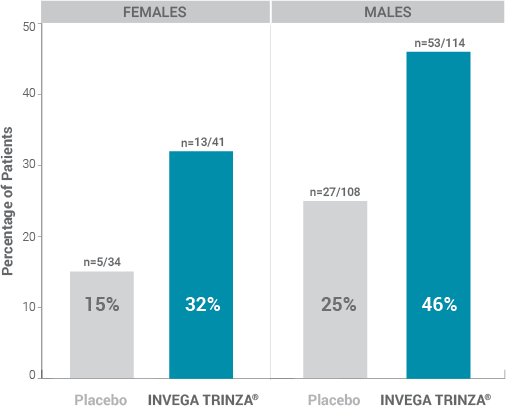
Prolactin Elevations1,2*
% of Patients with Potentially Prolactin-Related Adverse Reactions1,3†
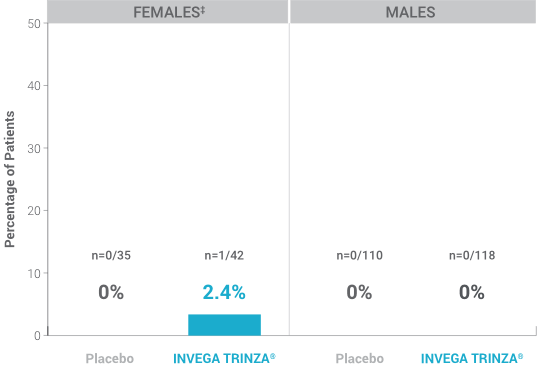
†Prolactin adverse reaction reports among all patients in the double-blind phase, regardless of baseline prolactin levels.
‡One female on INVEGA TRINZA® reported amenorrhea.
Observed in the Open-label Phase*§
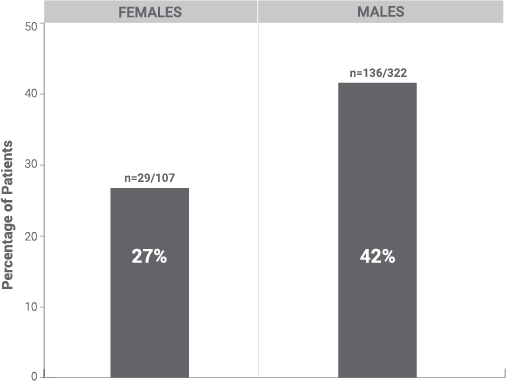
Prolactin Elevations1
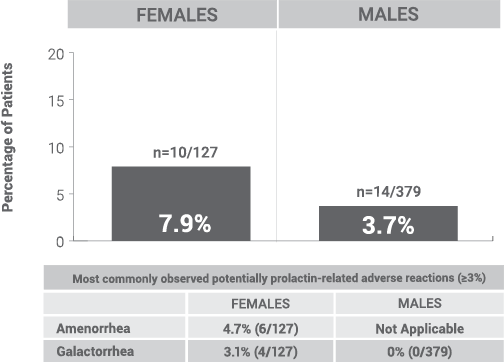
% of Patients with Potentially Prolactin-Related Adverse Reactions1,2||
*Elevations of prolactin to above the reference range (>13.13 ng/mL in males and >26.72 ng/mL in females) during the open-label phase relative to baseline.
§During the open-label phase, subjects received several doses of INVEGA SUSTENNA® (paliperidone palmitate) followed by a single dose of INVEGA TRINZA®.
||Prolactin adverse reaction reports among all patients in the open-label phase, regardless of baseline prolactin levels.
References: 1. INVEGA TRINZA® [Prescribing Information]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; August 2021. 2. Data on file. Janssen Pharmaceuticals, Inc., Titusville, NJ. 3. Berwaerts J, Liu Y, Gopal S, et al. Efficacy and safety of the 3-month formulation of paliperidone palmitate vs placebo for relapse prevention of schizophrenia: a randomized clinical trial. JAMA Psychiatry. 2015;72(8):830-839.