Clinical Data Demonstrating Time to Relapse With INVEGA TRINZA®
A Long-term Maintenance Study Demonstrated the Efficacy and Safety of INVEGA TRINZA® vs Placebo1,2
Abbreviated Study Design1,2
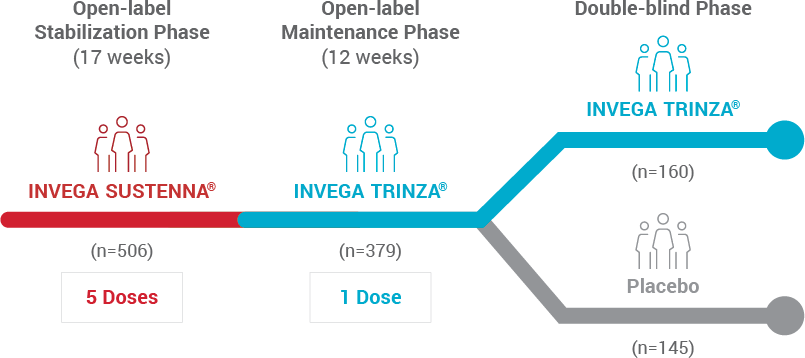
Following stabilization with INVEGA SUSTENNA® (paliperidone palmitate), all patients received 1 dose of INVEGA TRINZA® 12 weeks before randomization in the double-blind phase of the clinical trial.1,2
Study Design
A randomized, double-blind, placebo-controlled, long-term maintenance study compared 3-month paliperidone palmitate (INVEGA TRINZA®) with placebo in adults with schizophrenia. Patients were treated for 17 weeks with 1-month paliperidone palmitate (INVEGA SUSTENNA®) during an open-label, flexible-dose stabilization phase, and then a single dose of INVEGA TRINZA® during an open-label maintenance phase. This was followed by a fixed dose of INVEGA TRINZA® or placebo once every 3 months in a variable-duration, double-blind phase.1,2
Inclusion and Exclusion Criteria
Key Inclusion Criteria1,2:
- Adult patients (aged 18 to 70 years) with a diagnosis of schizophrenia* for ≥1 year before screening
- PANSS total score <120 at screening and baseline
- Patients with acute symptoms (if previously treated with oral antipsychotics) or who were clinically stable (if treated with long-acting injectable antipsychotics)
*Based on Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV-TR ®)
PANSS=Positive and Negative Syndrome Scale.
Key Exclusion Criteria2:
- Primary, active DSM-IV® diagnosis other than schizophrenia
- Significant risk of suicidal behavior
- History of substance dependence within 6 months before screening
- Involuntary status in a psychiatric hospital at screening
- History of neuroleptic malignant syndrome, tardive dyskinesia, or any malignancy in the previous 5 years, except basal cell carcinoma
In a Long-term Maintenance Trial, 93% of Adult Patients Treated With INVEGA TRINZA® Remained Relapse-free1
Primary Endpoint: Longer Time to Relapse vs Placebo1,2
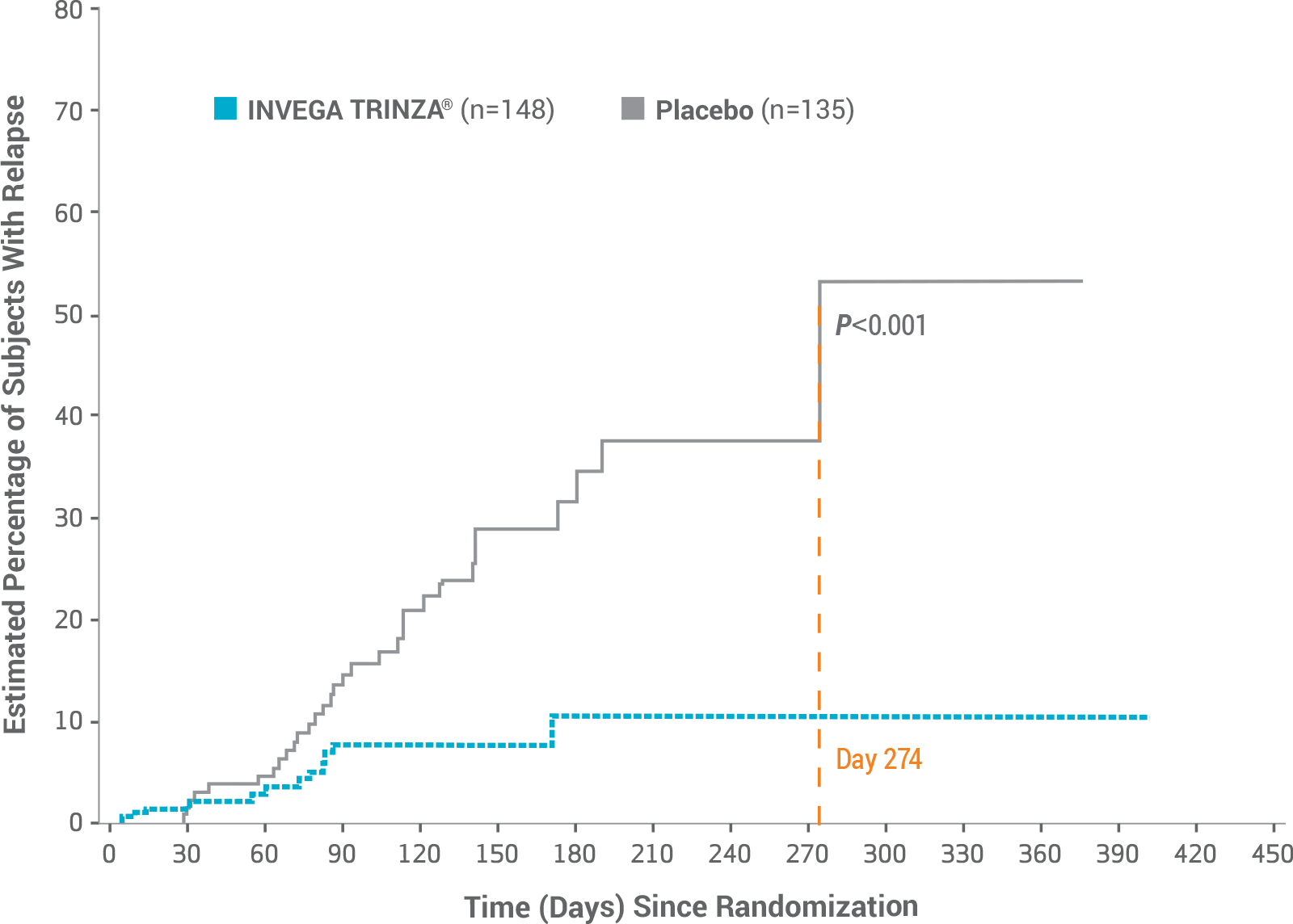
Key Study Observations1,2
- 7% of patients taking INVEGA TRINZA® relapsed vs 23% of patients taking placebo (P<0.001)1,2
- Median time to relapse for placebo was 274 days in the double-blind phase1,2
- Median time to relapse in the INVEGA TRINZA® arm could not be estimated due to low percentage (7.4%) of patients with relapse1,2
- Due to the significant efficacy of INVEGA TRINZA®, the study was terminated early at the preplanned interim analysis by an Independent Data Monitoring Committee1,2
The study protocol designated that an Independent Data Monitoring Committee perform an interim analysis after 42 relapse events had occurred across both arms of the trial. At that point, the committee decided to terminate the study because of the significant difference in efficacy favoring INVEGA TRINZA®, and the interim analysis became the primary efficacy analysis.1,2
Secondary endpoint: Change in mean PANSS total score over time2
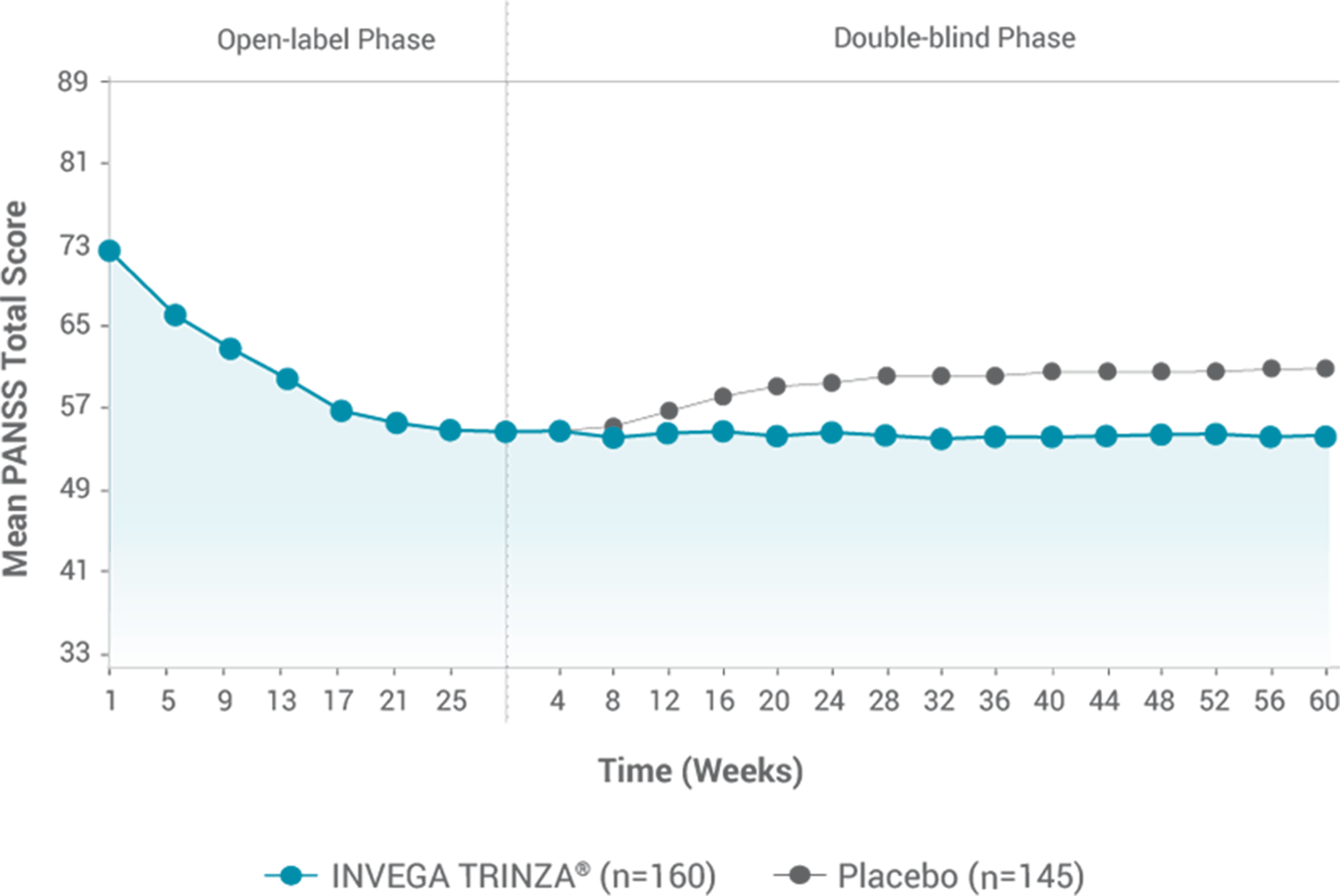
*Population included adults diagnosed within 5 years of study entry.
Relapse Criteria1,2
Relapse was defined as ≥1 of the following:
- Hospitalization for schizophrenia symptoms (involuntary or voluntary admission)
- 25% increase in PANSS total score from randomization on 2 consecutive assessments between 3 and 7 days apart for patients scoring >40 at randomization, or a 10-point increase of patients scoring ≤40 at randomization
- Increase in distinct PANSS item scores (P1, P2, P3, P6, P7, or G8) for 2 consecutive assessments between 3 and 7 days apart
- Clinically significant, deliberate self-injury or violent behavior resulting in suicide, injury, or significant damage
- Suicidal or homicidal ideation and aggressive behavior
Indicated for the treatment of schizophrenia in adults.
The full constellation of symptoms and the relevant diagnostic criteria should be consulted and are available in the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV® or current version) where applicable.
Study Phases
Stabilization Phase
A 17-week flexible-dose open-label transition period with 1-month paliperidone palmitate1,2 (first part of a 29-week open-label stabilization phase)
| 17 weeks | N=506 |
- Patients had to be clinically stable at the end of this period before receiving INVEGA TRINZA® at the week 17 visit
- Clinical stability was defined as achieving a PANSS total score <70 at week 17
Maintenance Phase
A 12-week open-label maintenance period with a single dose of INVEGA TRINZA®1,2 (second part of a 29-week open-label stabilization phase)
| 12 Weeks | n=379 |
- Patients received a single dose of INVEGA TRINZA®, which was a 3.5 multiple of the last dose of 1-month paliperidone palmitate
- Subjects had to remain clinically stable before entry into the next period (double-blind)
- Clinical stability was defined as achieving a PANSS total score <70 and scores of ≤4 for 7 specific PANSS items
Double-Blind Phase
A variable-length double-blind treatment period with INVEGA TRINZA®1,2
| INVEGA TRINZA® | n=160 |
| PLACEBO | n=145 |
- 305 stabilized patients were randomized 1:1 to continue treatment with INVEGA TRINZA® or placebo until relapse, early withdrawal, or the end of the study
- Patients were randomized to the same INVEGA TRINZA® dose received during the open-label phase (ie, 273 mg, 410 mg, 546 mg, or 819 mg) or to placebo, once every 3 months
- The mean duration of exposure during the double-blind phase was 175 days in the INVEGA TRINZA® group and 150 days in the placebo group
Early Termination Information
Independently Monitored Study1,2
- An Independent Data Monitoring Committee (IDMC) performed ongoing safety monitoring, an efficacy interim analysis, and provided recommendations about modifying, stopping, or continuing the study
- The IDMC consisted of 4 academic psychiatrists and 1 statistician who independently reviewed safety data on a quarterly basis, along with 1 planned unblinded efficacy analysis
Significant Efficacy Resulted in Early Termination1,2
- The protocol planned for an interim analysis after 42 relapse events and full analysis after 70 relapse events
- The interim analysis showed a significantly longer time to relapse in patients treated with INVEGA TRINZA® compared with placebo, and the study was stopped early by an IDMC because efficacy was demonstrated
References: 1. INVEGA TRINZA® [Prescribing Information]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; August 2021. 2. Berwaerts J, Liu Y, Gopal S, et al. Efficacy and safety of the 3-month formulation of paliperidone palmitate vs placebo for relapse prevention of schizophrenia: a randomized clinical trial. JAMA Psychiatry. 2015;72(8):830-839.